This week in class we worked more on why ice melts faster on metal than on wood. The forces between particles causes them to either speed up or slow down. For example: when playing pool, a ball is hit against another. What happens to the 1st ball when it hits the second? The 1st ball slows down and the second ball speeds up.
I was able to relate to what a bit of what I already know. I knew that ice melted faster on metal than wood but I never knew the actual reasoning as to why. After this lab, I now know why it happens and can understand why.
I will be using this in my future classroom because we can do experiments like this in class. Young kids are always asking questions and are being curious when it comes to things such as ice, and why it melts in our hands.
Question #1
What are the approximate melting points of the substances B and E?
This model can simulate a substance that does not have a melting point.
Find that substance and click Take snapshot to show what substance it is.
Describe why it does not have a boiling point, and how you know that it doesn't.
(You can use the drawing tool for your answer.)
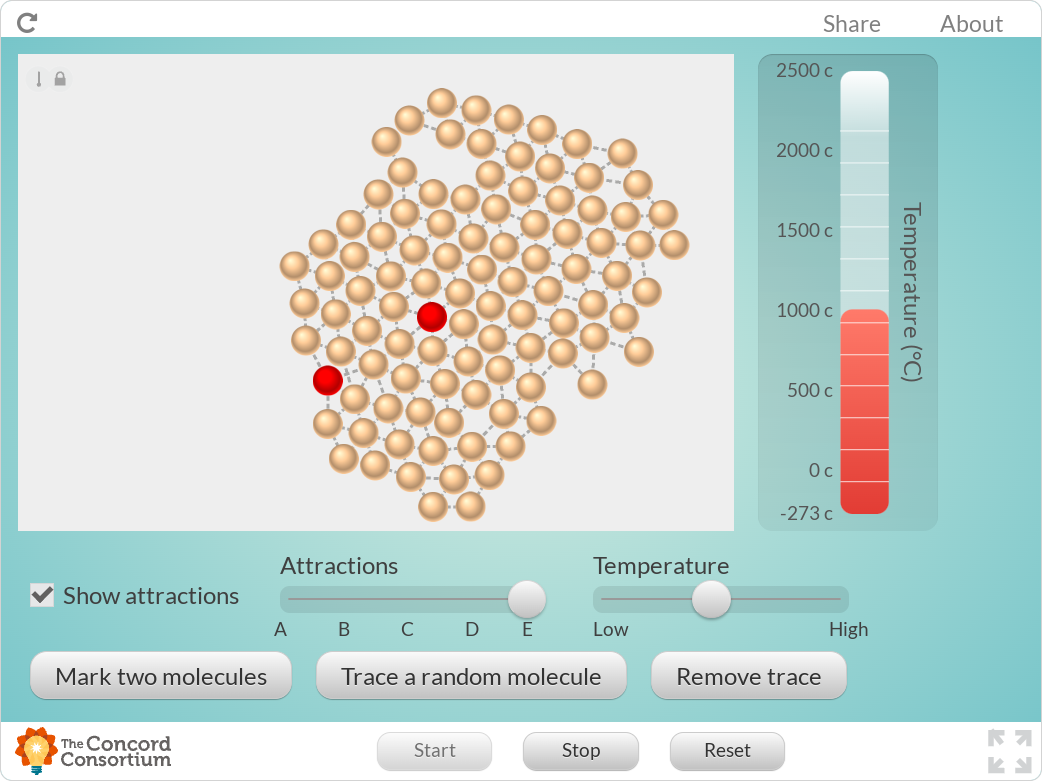
Make up a rule that describes how boiling point changes with the strength of particle attraction. Use evidence from the model to justify your rule.
What additional questions do you have about this model?
Hi Annette! I really liked your analogy when playing pool. I think that is a perfect description as to what is happening scientifically with all the molecules. Nice work!
ReplyDelete